On May 20, 2021, the European Commission published the final report on the implementation of the Tobacco Products Directive (TPD), COM(2021) 249 final, in its Official Journal (OJ), in which new tobacco products and other emerging products, e-cigarettes and liquid storage The implementation of containers and inhaled herbal products was notified and conclusions were drawn, which will serve as the basis for revising the relevant control provisions in the new TPD regulations.
What is the Tobacco Products Directive (TPD) and Article 20 Directive?
Interpretation of the Electronic Cigarette TPD Directive (2014/40/EU) Directive
1. The Tobacco Product Directive (TPD) is a regulation used to regulate and supervise the manufacture, sale, display (product design, packaging, etc.) of products and all tobacco and tobacco-related products (such as e-cigarette products).
2. At present, in most EU member states, electronic cigarettes are regulated and regulated as recreational consumer products. Starting from 2016, electronic cigarettes and related products will be regulated by Article 20 of the TPD.
3. On the surface, the Tobacco Products Directive (TPD) is designated for the smooth operation of tobacco products in the international market. In fact, many provisions of the TPD are set to reduce the attractiveness of tobacco products to achieve a higher standard of protection for human health. .
EU TPD Directive on Electronic Cigarette Content
1. The main contents of the product electronic notice should include:
(1) Manufacturer's name and contact information;
(2) Ingredient list and release content;
(3) Toxicological data;
(4) Information on nicotine dose intake;
(5) Description of product composition and production process;
(6) Statement of responsibility for product quality and safety.
2. Requirements for e-liquid and liquid reservoir:
E-liquid containing nicotine is only put on the market in professional fillers, and the volume of the filling container does not exceed 10mL, and in a single-use electronic cigarette or single-use pod, the volume of the pod or liquid reservoir must not exceed 2mL.
3. Requirements for nicotine:
(1) In the e-juice containing nicotine, the nicotine content shall not exceed 20mg/mL;
(2) Under normal conditions of use, the nicotine release of electronic cigarettes should be maintained at a stable level.
4. Preparation of documents before applying for TPD registration of electronic cigarettes:
(1) Production process documents;
(2) Release test report;
(3) Consistency detection method of nicotine release;
(4) Instructions for refillable atomizer open & refill.
5. Overview of the registration process (EU TPD)
(1) Electronic cigarette certification (ECAS) registration website in the EU TPD directive:
https://webgate.ec.europa.eu/cas/eim/external/register.cgi
(2) Apply for a Submitter ID;
(3) Fill in the form (about 10 working days to complete);
(4) After the registration is completed, the administrator will be notified by email, indicating that the ID registration is successful, and indicate the ECAS and Submitter ID;
(5) After success, log in to the account opening information platform (EU-CEG) to fill in the information, upload the attachments, and select different countries to submit the information separately.
6. Test requirements for electronic cigarette TPD
To test the content of the following chemical substances in the smoke released by electronic cigarettes, the first 3 items are not included in the test of e-liquid products, and all 7 items are tested for products containing e-liquid.
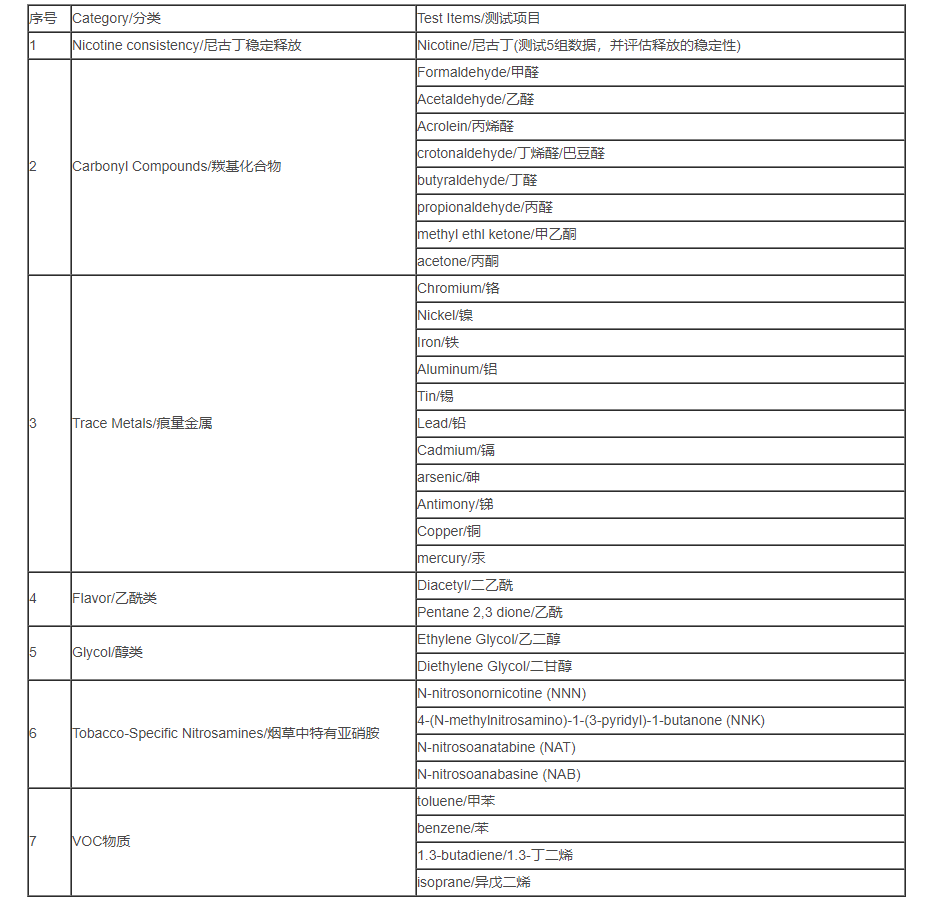
ZRLK testing can provide our customers with EU directive TPD certification service. If you want to know more about EU directive TPD certification requirements or have products that need EU directive TPD certification, please feel free to contact us, our engineers will be in The first time to serve you!













