On April 11, 2022, the European Commission published Regulation (EU) 2022/586 in its Official Gazette (OJEU), adding 5 substances to the list of authorized substances in REACH Annex XIV, which will take effect on the 20th day after publication in the Official Journal . By then, the list of authorized substances under the REACH regulation will increase to 59 items.
The new substances added to Annex XIV (List of Authorized Substances) of the REACH Regulation are as follows:
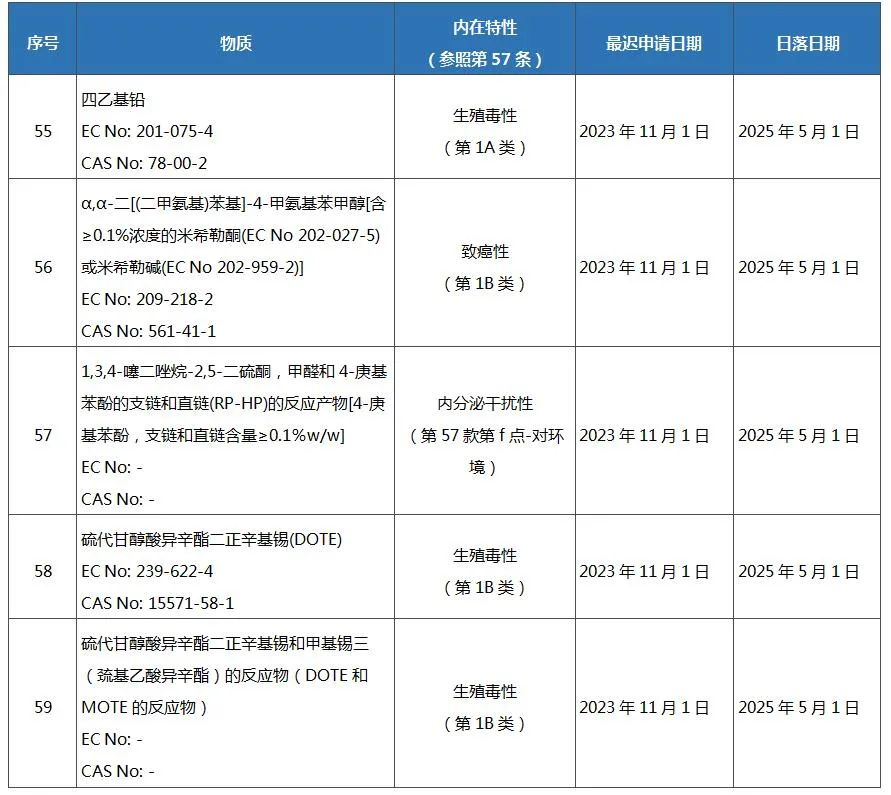
Once a substance is added to the REACH Annex XIV List of Authorized Substances, from the "sunset date" (that is, the date on which the placing on the market and use of the substance is prohibited unless authorized), only authorized operators, or operators at the latest The substances may only be placed on the market or used in the EU if an application for authorization has been submitted before the late application date, but a decision on the application has not yet been taken.
EU Authorization List Background
Authorized substances are substances that require authorization before they can be placed on the EU market or used. The authorizations in the REACH Regulation are for the identification and management of Substances of Very High Concern (SVHC). SVHCs that require authorization will be added to REACH Annex 14, the "authorization list". Each entry in Addendum 14 has a "sunset day", and generally speaking, substances added to Addendum 14 cannot be used after sunset unless exempted or authorized. Manufacturers, importers or downstream users can apply for the authorization of the corresponding substances. Imported items do not need to consider authorization requirements, but need to consider the requirements related to Candidate List substances and restrictions in Appendix 17.













