On June 16, 2020, the European Union Chemicals Agency (ECHA) added 4 substances from the 23 batch of 5 review substances to the SVHC candidate list. Since the committee of the member states did not agree on the determination of resorcinol (EC 203-585-2; CAS 108-46-3) as SVHC proposed by France, resorcinol was not included in SVHC for the time being, but Subsequent European Commission will still further confirm this substance, so as to make a final decision. So far, the EU's list of highly concerned substances (SVHC) has been increased to 209 items.
The European Chemicals Agency (ECHA) will update the SVHC candidate substance list in late June.
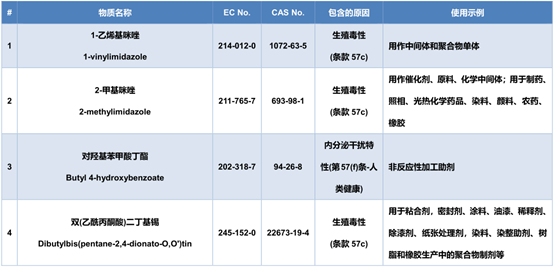
The newly added 4 items of SVHC candidate substance information are as follows:
The REACH regulation stipulates that when the product contains SVHC substances and the content exceeds 0.1%, it is necessary to notify ECHA or carry out information transmission within the supply chain; when the SVHC content in the article is greater than 0.1% and the total amount of SVHC produced/imported in the article each year When it exceeds 1 ton, the manufacturer or importer must notify the European Chemicals Agency (ECHA) within 6 months after the new substance is included in the list.
With the continuous updating of the SVHC candidate list, enterprises are facing more and more control requirements. Enterprises need to raise the risk awareness of their products. Within six months after the substance is included in the SVHC candidate list (from June 16, 2020), eligible companies need to complete the SVHC notification in the article. It is recommended that companies investigate the supply chain as early as possible to calmly respond to changes in regulations.
Related tips
1. What is the REACH directive?
The REACH directive is the English abbreviation for "Registration, Evaluation, Authorization and Restriction of Chemicals". The main content of REACH is to prove that daily-use products do not contain harmful chemicals. Therefore, all daily-use products manufactured in the EU or imported into the EU market must pass the registration, inspection and approval of the content of hazardous chemicals, and once they exceed the prescribed content, they cannot be sold on the EU market.
2. What is a highly concerned substance?
SVHC, which is a substance of great concern, comes from the EU REACH regulation. According to Article 57 of REACH, substances that may have a serious impact on human health or the environment during daily use will be included in the candidate list, and this list is called List of substances of high concern. Once they are included in the authorization list, the industry needs to apply for a permission to use the substance after the restriction is implemented.
3. How to deal with the "substance" exceeding the standard?
If the "article" products placed on the EU market contain any SVHC candidate substance with a concentration of >0.1%, the EU manufacturer or importer should perform the notification and notification obligations stipulated in Reach regulations: ?
(1) When receiving consumer inquiries, relevant information must be provided to consumers within 45 days;
(2) When the content is> 0.1% and the total amount of the substance entering the EU is> 1 ton/year, the EU manufacturer or importer must first notify ECHA before the product can be sold in the EU market; ?
(3) If the "substances or mixtures" products (such as paints, inks, etc.) contain SVHC candidate substances, the manufacturer should provide the downstream user with a safety technical specification (SDS).
note:
Unqualified products exported to the EU will be punished. EU customs will refuse to import the product, seize the goods, etc., and may face high fines.
Our company provides related testing and certification services, please contact us for more information.