In June 2021, the European Commission proposed to amend REACH Regulation (EC) No 1907/2006, adding 5 new substances to REACH Regulation Annex XIV (List of Authorized Substances).

In the form of Annex XIV of REACH, it is proposed to add substances 55 to 59:
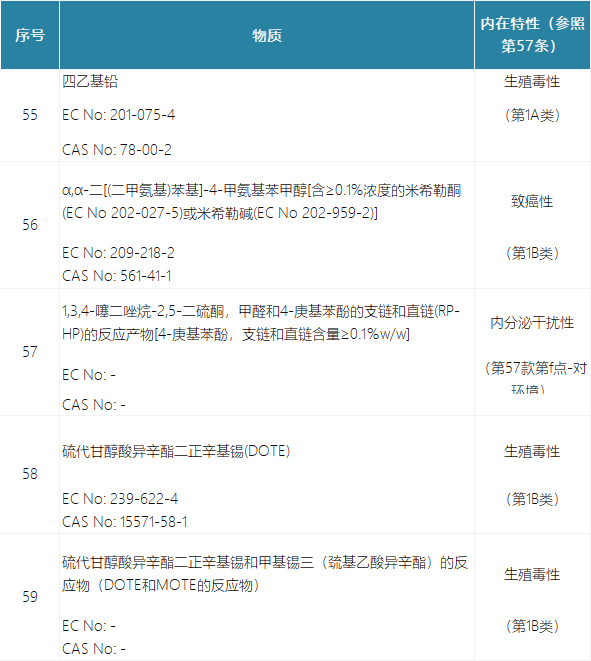
The latest application date is proposed to be 18 months after the amendment takes effect, and the sunset date is proposed to be 36 months after the amendment takes effect.
Once the draft regulations are passed and come into force, after the sunset date, only authorized operators, or operators who have submitted an authorization application before the latest application date, but have not yet made a decision on the application, can enter the EU Put these substances on the market or use them.












